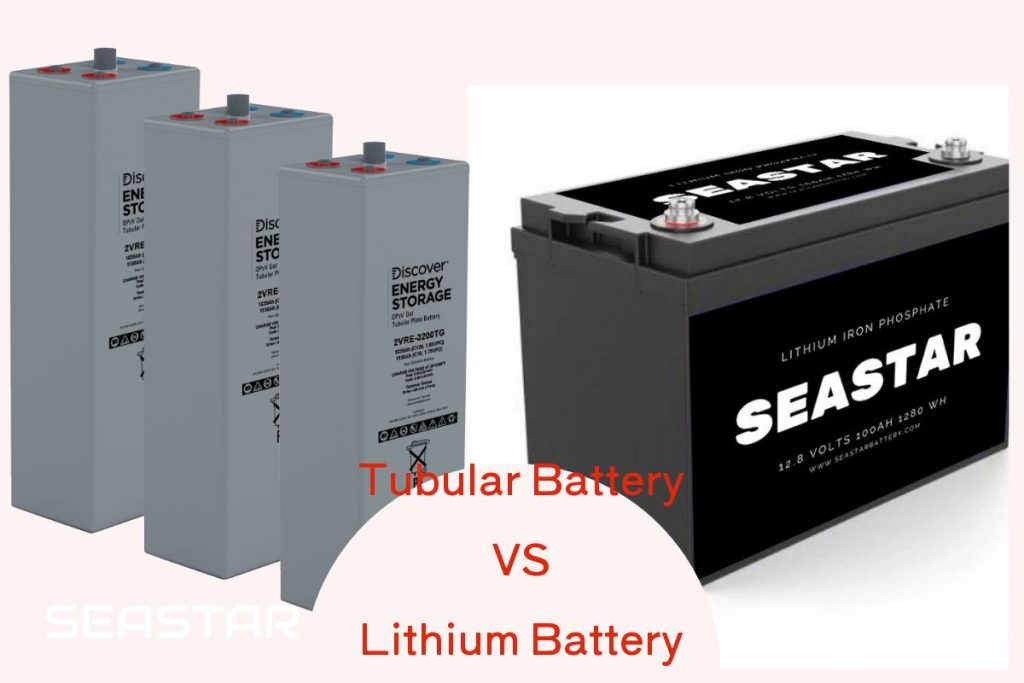
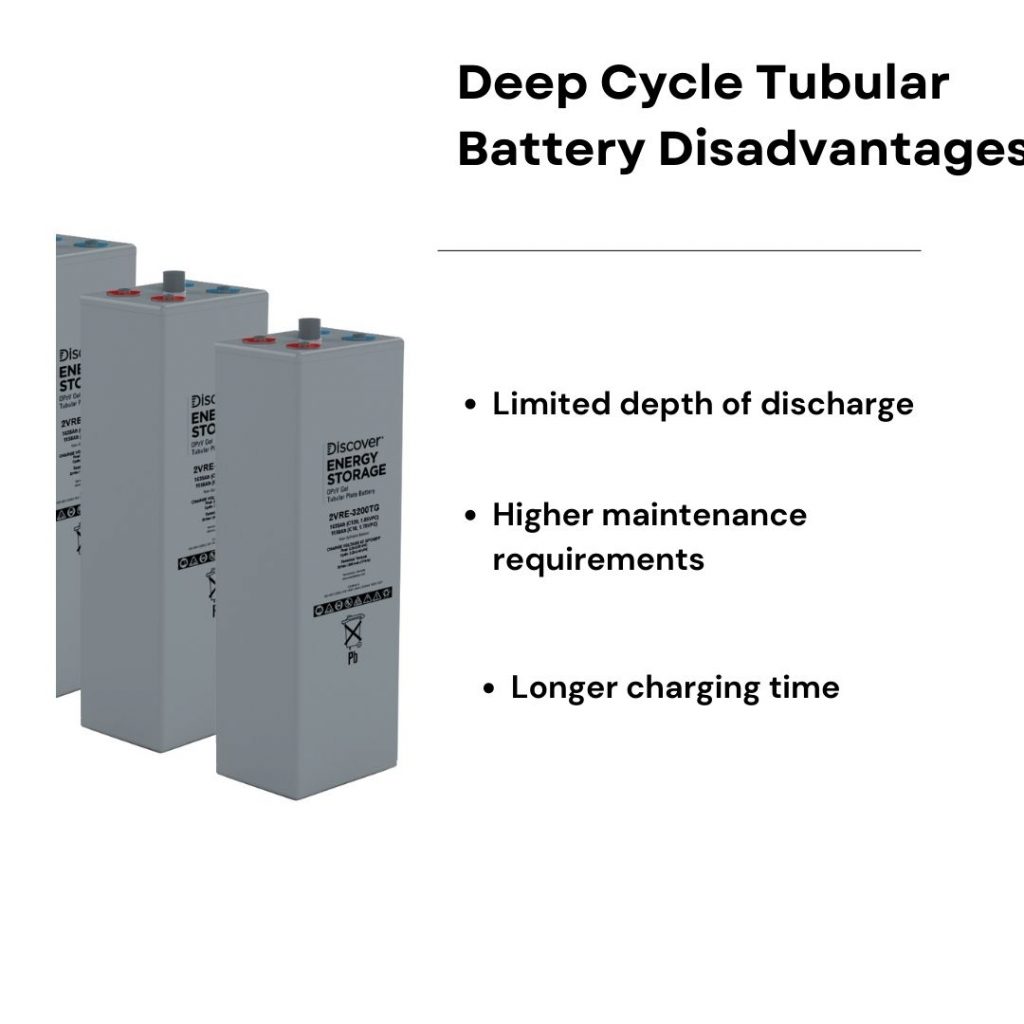
Discover the Future of Aircraft Batteries with Seastar Battery
Unlock unparalleled performance and reliability for your aircraft with Seastar Battery’s cutting-edge lithium batteries. As a leading manufacturer based in Shenzhen, China, we pride ourselves on delivering top-notch solutions to international procurement and import businesses in the aviation industry.
Table of Contents
State of the Art Technology and Expert Engineering
Experience the power of advanced technology and industry expertise as we design and produce high-performance aircraft batteries. Our skilled team of engineers meticulously crafts each battery to meet the strict safety and quality standards set by aviation authorities.

Tailored Solutions for Your Unique Power Needs
No two aircraft are the same, which is why we offer customized battery solutions for a wide range of aircraft models. With our extensive experience, we understand the specific power requirements of different aircraft and ensure optimal performance and reliability.

Certified to Soar Above Safety Standards
Safety is of utmost importance in aviation. Rest assured that our batteries undergo rigorous testing to meet and exceed global aviation regulations. We proudly hold essential certifications such as FAA, EASA, and ISO 9001, demonstrating our commitment to the highest safety standards.

Reliability When It Matters Most
Experience peace of mind knowing that our aircraft batteries are built to deliver unwavering reliability, even in the most demanding conditions. From engine starting to powering onboard systems, our batteries are engineered to perform flawlessly in critical operations.
Leading the Charge in Sustainability
Join our mission to protect the environment with our eco-friendly manufacturing practices. Seastar Battery is dedicated to reducing our carbon footprint and providing sustainable battery solutions for the aviation industry.

Aircraft Battery Manufacturers
We are a specialized company dedicated to aircraft battery manufacturing, boasting years of experience and technical expertise. As a leading player in the aircraft battery industry, we are committed to providing high-quality, reliable, and safe battery solutions for the aviation sector.
Trust in Seastar Battery
As your trusted aircraft battery manufacturer, we are here to meet the needs of international procurement and import businesses. Our cutting-edge technology, expertise, safety certifications, reliability, and sustainability initiatives empower the aviation industry. Choose Seastar Battery for superior performance and peace of mind. Contact us today to find the perfect battery solutions for your aircraft fleet.
Discover the Future of Aircraft Batteries with Seastar Battery Read More »